Temple University Hospital (TUH) is participating in a nationwide, Phase III clinical trial that could bring new hope and a new treatment to heart failure
patients who have exhausted all surgical options.
“This trial has the potential to be a game changer for these patients,” says Howard A. Cohen, MD, local Principal Investigator for the trial and Director
of Interventional Cardiology and the Cardiac Catheterization Laboratories at TUH. “You’re talking about patients who could go from being bedridden because
they get so breathless to now being able to walk around and enjoy normal day-to-day living,” adds Dr. Cohen, who also serves as Director of the Temple
Interventional Heart & Vascular Institute, and Professor of Medicine at Temple University School of Medicine (TUSM).
The trial – called the Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy for High Surgical Risk Patients (COAPT) – is investigating the
safety and effectiveness of the MitraClip device in heart failure patients who have functional mitral regurgitation (MR) and are considered extremely
high-risk for surgery.
Functional MR is characterized by a mitral valve that leaks due to it not closing completely. It doesn’t close completely because the patient’s poorly
functioning left ventricle is enlarged, but the valve itself has retained its normal size. Together the poor left ventricle function and leaky mitral valve
combine to lower the heart’s ejection fraction, which is the amount of blood pumped out of the ventricle with each heartbeat.
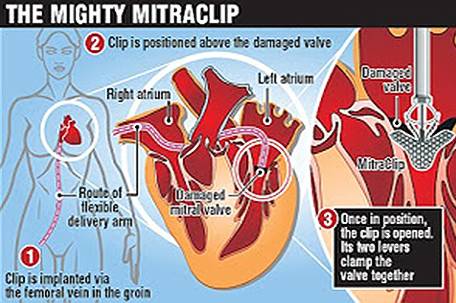
The MitraClip device is an implant designed to be a minimally invasive method of mitral valve repair. The device “captures” the mitral valve and clips it
so that the size of the valve opening is reduced, thus allowing it to close more completely and eliminate leaking.
While the patient is under general anesthesia, the device is implanted by Interventional Cardiologists using a catheter inserted percutaneously through a
vein in the leg. The Interventional Cardiologist uses three-dimensional echocardiography to carefully maneuver the MitraClip into the heart and into place
above the leak in the valve. Then, the clip is advanced into the left ventricle below the valve leaflets, retracted, and closed to hold the leaflets
together. The end result is a double orifice opening that allows blood to flow on both sides of the clip.
Patients enrolled in the trial will be randomized to either receive the MitraClip device and best medical care, or to receive best medical care alone.
Those that receive the device will have the MitraClip implanted and then remain in the hospital for one or two days.
Patients will be monitored for two years following the procedure. During that time, doctors will perform follow up echocardiograms to monitor whether
“reverse remodeling” takes place – meaning the heart gets smaller and its function and ejection fraction improves. Doctors will also note any heart failure
re-hospitalizations.
The results of patients who receive the MitraClip device will be compared to those who do not receive the device. The primary measure of success for the
trial will be whether patients who receive the device experience less heart failure re-hospitalizations than those who do not receive the MitraClip.
“There are a lot of patients out there suffering from severe mitral regurgitation,” says Dr. Cohen. “Our hope is that this device can provide them a
minimally invasive option to improve the heart’s function and the patient’s quality of life.”
Temple is currently screening patients for the COAPT trial. Interested patients or physicians may call Robin Vogel, Structural Heart Disease Program
Manager, at 215-756-6082 for more information.













Leave a Comment